By Ziyang Wang and Longqing Chen
Summary
The events that unfolded in 2020 rang an alarm bell for humanity: as of now, Covid-19 has claimed 2.8 million lives worldwide (“Covid-19 Dashboard”). Major businesses across the globe found themselves in deep financial trouble: already under the great distress of debt before the pandemic, the American rental car giant Hertz had to file for chapter 11 bankruptcy to keep existing operations running (“Hertz Bankruptcy”). Smaller businesses, on the other hand, fared much less fortunate: approximately one out of five open businesses last January have completely halted their transactions. What’s worse, according to the Wall Street Journal’s prediction:, most of these closing small businesses are closing for good (“Covid Crushing Businesses”). As a result, the stock market became more volatile than ever. The US market circuit breaker was first put in place after the 1987 market crash. Since then, it has been triggered five times: four of which happened during March 2020 (“March Madness”).
The first purpose of this memorandum is to delve deep into common issues that the global community has to deal with when battling widespread diseases. Then, it will illustrate how pharmaceutical outsourcing strategies may overcome these issues. Finally, we shall offer several economic policy alternatives for governments around the world to encourage pharmaceutical outsourcing. We will conclude by proposing a multinational IP Flexible and De-linkage hybrid approach for policymakers around the world.
Issue
The world is not prepared for widespread diseases.
It takes very long for medicines to be developed. Traditionally, the research process of discovering a safe and effective vaccine takes two to five years. According to Wellcome Trust, a charitable foundation based in London, it would take more than 10 years in total for a reliable vaccine to fully develop (“Vaccine Development Barriers”). Jennifer Pancorbo, director at the NC State Biomanufacturing Training and Education Center, explained clearly to us why that is the case: everything takes time, and that includes understanding the disease and growing microorganisms (“Vaccine Q&A”) The perfect example is the history of polio: though the disease was first discovered in 1894, it wasn’t until half a century later (1953) that the first effective vaccine was developed (“Polio Vaccine”). An even more modern example is the case of antimicrobial resistance (AMR): if not curbed, it could be the cause of around 10 million deaths yearly by 2050 (O’Neill 11) Unfortunately, only one novel class of antibiotics has been developed in the past 40 years besides bedaquiline (Hamelmann et al. 14).
Patient recruitment is another vital factor in determining clinical trial timings and whether new drugs can be launched quickly (“CRO Sector”). One of the biggest challenges to developing medicines and vaccines for widespread diseases through clinical trials is the varied consequences these diseases unfold on varied demographic groups. There are so many independent variables that the researchers need to test out: for instance, how Covid-19 expresses itself on different patients depends on many factors such as age, underlying conditions, region, and so on. This requires a large number of clinical trials to be conducted. Nonetheless, the peculiar nature of Covid-19 rendered the pool of eligible patients very small. On one hand, given the fatal nature of Covid-19 on patients with underlying conditions, very limited clinical trials on this population could continue since clinical resources need to be reallocated to medical care (“Ethical Challenges”). On the other hand, about 80% of the patients could recover before their condition becomes more serious (“Recruitment Challenges”). This dichotomy proved to be a great challenge for researchers who wanted to test out potential therapies that could mitigate more serious Covid-19 conditions.
What’s more, even if the medicine is developed, manufacturers cannot produce the medicine at a fast pace. The first bottleneck for manufacturers is the lack of sufficient resources. In the case of Covid-19 vaccine production, the borosilicate glass and rubber used in vaccine packaging are in dire shortage. Another bottleneck for manufacturers is quality control. According to Pancorbo, three key elements need to be considered before distributing each batch of vaccines: identity, purity, and potency (“Vaccine Q&A”). To make sure every vial of vaccine is safe and effective, every criterion is of utmost importance. For instance, the Moderna vaccine has very strict temperature requirements (it needs to be stored between -13F to -8F), and hundreds of thousands of these vaccines wind up in the garbage because they were not stored at the right temperature. Just this January in Maine, 4,400 doses of the Moderna vaccine were compromised for this reason (“Maine CDC”).
Solution
Outsourcing is the business practice of hiring a party outside a company to perform services and create goods that traditionally were performed in-house by the company’s own employees and staff (“Why Outsourcing”) Evidently, pharmaceutical outsourcing refers to pharmaceutical companies’ practice of contracting parts of their business functions to other organizations/companies. This practice resolves the three problems mentioned above.
Pharmaceutical outsourcing is a great catalyst for innovation. Exploiting comparative advantages is often the first thing that comes to our minds when outsourcing is mentioned. Nonetheless, it is far from being the only function of outsourcing: when examining the impact of exploitative and explorative knowledge sharing on the performance of long-term R&D outsourcing relationships, Im and Rai demonstrated that companies could benefit from leveraging existing competencies as well as pursuing new learning opportunities through exchanging knowledge in interorganizational relationships (1281). This benefit is further corroborated by a study on UK manufacturing firms: the authors observed that domestically owned multinational enterprises received much higher R&D returns than domestic-based firms.
Similar for pharmaceutical firms, R&D outsourcing could be a powerful method for expanding their medical product pipelines. Gilmartin, when he was still the CEO of Merck back in 2002, celebrated the 11 potential treatments that are expected to debut in the coming years. However, by 2003 only two treatments were launched in time. The rest were either delayed or canceled. In hope of pulling its innovation pipeline out of deep trouble, Merck acquired Aton Pharmaceuticals Inc (Higgins and Rodrigues 355). The WSJ later described this acquisition as helpful in “enhancing its internal research efforts to develop potential new medicines for the treatment of cancer” (“Merck to Buy Aton”)
More outsourcing means more clinical trial opportunities. Conducting clinical trials with appropriate participant sample sizes is extremely crucial in reaching the goals of medical researches (Julious 25). Unfortunately, due to the difficulties related to patient recruitment, many trials often struggled to meet their target sample size within the timeline and budget originally planned (McDonald et al. 9): from 2001 to 2005, 51% of multicentered trials reported to have struggled in patient recruitment (McDonald et al. 166). Pharmaceutical outsourcing may be a great way to combat this issue: Jones and Minor discovered by granting firms access to a vast population of patients from various locations, outsourcing may help firms increase the number of clinical trials conducted, thereby adding a 20% in productivity per full-time equivalent (Minor et al. 77).
In fact, some pharmaceutical firms have already greatly, if not entirely, shifted their strategy to outsourcing when dealing with patient recruitment. The results are phenomenal.
Labcorp, the world-renowned clinical services provider, acquired Covance and Chiltern in 2015 and 2017 respectively in hope of improving patient recruitment and trial efficiency. As a result of these deals, Labcorp not only further solidified its geographic presence but also strengthened its position as the largest CRO in the industry due to the expanded clinical outsourced services (“CRO Sector”). Just this February, the eminent pharma services company NeoGenomics announced its partnership with Praxel, another giant American provider of biopharmaceutical services in hope of improving clinical trials. Explaining the company’s motives, a Praxel representative commented:
“As a diagnostic lab that runs approximately 1 million oncology tests annually, NeoGenomics was an attractive partner whose resources and expertise could be leveraged across multiple applications…” (“Parexel-NeoGenomics Partner”)
Finally, pharmaceutical outsourcing allows for the optimization of resource utilization, thereby increasing manufacturing capacity. As Higon et al. mentioned, “…through the utilization of R&D outsourcing strategy in local collaborative relationships, (exploitation-orientation) subsidiaries can acquire, identify, assimilate and access the information on the local market to improve the efficiency of modifying their existing production and process and to maximize their asset utilization in the local market, which in turn will enhance their productivity.” (Anon Higon, Manjon Antolin, and Manez 2011) (Chuang et al. 70).
No firm can serve as a better example than Pfizer.
Before the pandemic and beginning from 2008, Pfizer did a great job with this. Pfizer has outsourced many of its business functions such as data entry, monitoring, statistics, and medical writing to various third-party contractors. Its business model put so much emphasis on outsourcing to the point that it no longer handles any data management in-house (“Pfizer’s Novel Approach”). During the pandemic, Pfizer became even more engaged in the process of outsourcing and contracted its supply functions to more than 200 outside contractors (“Pfizer to Outsource”). Thanks to these measures, Pfizer claims it has managed to “align functionality, improve quality, and reduce cycle times” and will produce 1.3 billion doses of vaccine in 2021 (“Covid Vaccine Tracker”).
Policy Alternative
The current patchwork of public, private and philanthropic funding is inadequate to sufficiently and sustainably expand access to health technologies. Both the public and private sectors must make and coordinate larger and more sustainable financial commitments to achieve maximum utility and effect (Hamelmann et al. 8). By encouraging pharmaceutical outsourcing, governments around the world can improve global communities’ ability to battle health emergencies and to control the spread of epidemics, such as Covid-19, Ebola, HIV infection, malaria, tuberculosis, and other diseases. Nonetheless, this is not an easy task to accomplish due to intellectual property security concerns.
With an R&D expenditure of $79.6 million in 2018 alone, the pharmaceutical industry has the highest R&D intensity in the US and the EU (Kermanimojarad 1). When outsourcing occurs in a knowledge-intensive sector such as this one, large amounts of advanced knowledge and technologies are shared between the customer and the vendor. Many feared the consequences, for instance: the infringement of IP may lead to catastrophic financial losses. James Love, the director of Knowledge Ecology International, explained this situation brilliantly in one of his articles published on IP Watch: “When we grant monopolies on products, through patents or other measures, the company that has the monopoly exploits the monopoly, fairly predictably, to maximize profits, and increasingly, this means aggressive pricing. Why do we have public policies to create monopolies? Because that is part of our system of funding R&D.”(“Delinkage of R&D Cost”).
Simply put, to encourage pharmaceutical outsourcing, there are two crucial things that need to be done. Firstly, a robust instrument needs to be put in place to break the lasting doxa of the “monopoly system”. After that’s done, we need to look for other equally viable sources of reward mechanisms for pharmaceutical R&D in replacement.
1. “Doxa breakers”
Developing countries could utilize WTO’s compulsory licensing and establish framework agreements to ensure IP Flexibilities, thereby widening the leeway for outsourcing.
At the World Trade Organization (WTO) Ministerial Conference in Doha in 2001, Trade Ministers signed a declaration stating their shared understanding of the terms of Trade Related Aspects of Intellectual Property Rights (TRIPS) (“Ministerial Declaration”), which used to guarantee exclusive marketing rights and product patents to medical innovators. Included in this declaration is the general consensus that when public health interests demand it, especially in developing countries, the TRIPS Agreement should be adapted in specific ways that ensure universal access to drugs. These adaptations later came to be known as the “TRIPS flexibilities” (“Implications”).
One of the most effective adaptations of the TRIPS flexibilities is compulsory licensing, which backs the government use of IP for non-commercial purposes, non-exclusive protection of test data, and parallel imports (Correa 1). So far, it has been used primarily in the forms of expanding imports or lowering drug price in HIV/AIDS-related treatments in Brazil, Ecuador, Ghana, Indonesia, Malaysia, Rwanda, Zambia, Zimbabwe, etc.
In 2006 and 2008, Thailand’s government has agreed to give government use licenses to allow the import and local manufacturing of generic versions of seven patent-protected medicines: Antiretroviral(ARV) drug, Efavirenz(EFV), the second-line ARV combination of lopinavir/ritonavir (LPV/r) and clopidogrel, letrozole, docetaxel, erlotinib, and imatinib, the last four being cancer drugs (Wibulpolprasert 11). By doing so, the Thai government significantly lowered drug prices and increased imports when negotiating with Merck (EFV for US$500 per patent per year(PPPY) to US$224 PPPY) and Abbot (LPV/r for US$2200 PPPY to US$676 PPPY) (Ford et al. 21).
During the Covid-19 pandemic, The Canadian Patent Act was amended by Canada’s COVID-19 Emergency Response Act to allow for a faster framework for obtaining a compulsory license on public health grounds. The amendment allows the government to grant necessary innovations a license and later negotiate compensation (Wong 3).
Another highly useful adaptation of the TRIPS flexibilities is the establishment of framework agreements – i.e. arrangements between one or more buyers and one or more suppliers that provide the terms governing contracts to be established for a certain period of time (“Framework Agreements”).
Not only do framework agreements strengthen the cooperation and avoid competition for pharmaceutical resources among developing countries, but they also send collective signals to companies in developed countries that are developing new drugs and medical services based on the positive commons approach, which would further encourage these companies to innovate drugs and medical services to meet the agreement Member’s most urgent needs.
What’s more, as pharmaceutical companies distribute their services at lower prices in accordance with these agreements, they also acquire the privilege to make binding deals with the beneficiaries and gain a more specific understanding of the demands of their products.
2. The Alternative Reward Mechanism
Initiating and financing Public-private partnerships (PPPs) is a great way to incentivize the de-linkage of pharmaceutical product price and R&D cost. According to the comparative analysis by Moran et al, in the field of neglected diseases, PPPs financed by the US government, philanthropic organizations, and major multinational firms are more effective, faster, and deliver better results than R&D centered in pharmaceutical corporations (“Neglected Disease Research”).
In contrast to pharmaceutical companies, PPPs operate on direct donations and for-profit settings according to different incentives and structures with a primary focus on risk-limiting, collaboration-centered IP management. This significantly lowers the cost of knowledge protection and facilitates innovation within the consortia (“Recalibrating Intellectual Property Rights”).
Another reward mechanism that helps with de-linkage comes with a specific outsourcing model: the Carrot model. By sharing IP with a third-party vendor, R&D costs can be partly supported by licensing revenue, therefore (to some extent) delinking the cost of research and the price of the product. As Choi et al. (2004) pointed out, as long as IP holders understand the complexity of licensing relationships and manage them effectively, the sharing of IP during outsourcing can actually be quite rewarding for both the customer as well as the vendor. In particular, the Carrot model of IP management in outsourcing is particularly helpful in reducing the R&D cost: instead of keeping the technical know-how and other IPs in-house as a way to prevent infringements on advanced knowledge, many firms after the 1990s actually chose to voluntarily share the IPs with other firms. By doing this, many firms were able to capitalize on the value of IPs before they depreciate in the industry and in the long run, speed up and make cheaper their new R&D activities (Choi et al. 41).
Recommendation
Based on the discussion above, this memorandum proposes a multinational IP Flexible and De-linkage hybrid approach for policymakers. This approach calls for developing countries to better utilize their IP flexibilities granted by the Doha Declaration, specifically compulsory licensing and framework agreement, so as to ensure that outsourcing can happen in dire situations regardless of IP protections. In addition, it suggests that developed countries should pour more government revenue into the funding of PPPs and adopt the Carrot outsourcing model so that new reward systems for pharmaceutical R&D could be established.
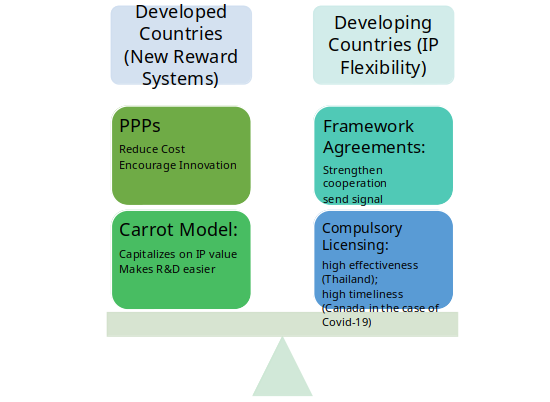
Figure 1 The Multinational IP Flexible and De-linkage Hybrid Approach
Bibliography
“Amendment of the TRIPS Agreement.” WTO, www.wto.org/english/tratop_e/trips_e/amendment_e.htm.
“CRO Sector M&A Drivers and Market Trends.” Results Healthcare, resultshealthcare.com/insight/cro-sector-drivers-and-market-trends/.
“Global Trade Update (October 2020).” UNCTAD, Oct. 2020, unctad.org/webflyer/global-trade-update-october-2020.
“More Than 652 Million Shots Given: Covid-19 Tracker.” Bloomberg.com, Bloomberg, 3 Apr. 2021, www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/.
“OCT Clinical Trials – The Leading CRO in Eastern Europe.” OCT Clinical CRO – Managing Clinical Trials in Eastern Europe, 23 Aug. 2020, oct-clinicaltrials.com/resources/articles/recruitment-challenges-for-covid-19-and-other-infectious-disease-trials.
“WORLD TRADE ORGANIZATION.” WTO, 14 Nov. 2001, www.wto.org/english/thewto_e/minist_e/min01_e/mindecl_e.htm.
Barnes, Kirsty. “Pfizer’s Novel Approach to Outsourcing.” Outsourcing, William Reed Business Media Ltd., 19 Apr. 2007, www.outsourcing-pharma.com/Article/2007/04/19/Pfizer-s-novel-approach-to-outsourciNg.
Bierer, B.E, et al. “Ethical Challenges in Clinical Research During the COVID-19 Pandemic.” Journal of Bioethical Inquiry, Springer Singapore, Dec. 2020, www.ncbi.nlm.nih.gov/pmc/articles/PMC7651825/.
Broom, Douglas. “5 Charts That Tell the Story of Vaccines Today.” World Economic Forum, 2 June 2020, www.weforum.org/agenda/2020/06/vaccine-development-barriers-coronavirus/.
Bubela, Tania, et al. “Recalibrating Intellectual Property Rights to Enhance Translational Research Collaborations.” Science Translational Medicine, American Association for the Advancement of Science, 22 Feb. 2012, stm.sciencemag.org/content/4/122/122cm3.full?sid=42f591b5-252b-4c95-a369-de6f3d958a2e.
Choi, Thomas Y., et al. “Intellectual Property Management: a Knowledge Supply Chain Perspective.” Business Horizons, vol. 47, no. 1, Elsevier Inc, 2004, pp. 37–44, doi:10.1016/j.bushor.2003.11.006.
Chuang, Wen-Bin, et al. “Does Stronger Intellectual Property Rights Protection Matter in Developing Local R&D Outsourcing Strategy?” Asia-Pacific Journal of Accounting & Economics, vol. 24, no. 1-2, Routledge, 2017, pp. 68–82, doi:10.1080/16081625.2016.1158658.
Correa CM, ‘Implications of the Doha Declaration on the TRIPS Agreement and Public Health’ (2002) WHO Health and Economics and Drugs EDM 12.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021, coronavirus.jhu.edu/map.html.
Douglas Broom, Senior Writer. “5 Charts That Tell the Story of Vaccines Today.” World Economic Forum, 2 June 2020, www.weforum.org/agenda/2020/06/vaccine-development-barriers-coronavirus/.
Dow Jones Newswires. “Merck to Buy Aton Pharmaceuticals.” The Wall Street Journal, Dow Jones & Company, 1 Mar. 2004, www.wsj.com/articles/SB107757665149537025.
Ford, Nathan, et al. “Sustaining access to antiretroviral therapy in the less-developed world: lessons from Brazil and Thailand.” Aids 21 (2007): S21-S29.
Framework Agreements, 2012, tfig.unece.org/contents/framework-agreements.htm.
General Council. “WORLD TRADE ORGANIZATION.” WTO, 6 Dec. 2005, www.wto.org/english/tratop_e/trips_e/wtl641_e.htm.
Hartman, Travis. “March Madness.” Reuters, Thomson Reuters, 19 Mar. 2020, graphics.reuters.com/USA-MARKETS/0100B5L144C/index.html.
Higgins, Matthew J., and Daniel Rodriguez. “The Outsourcing of R&D through Acquisitions in the Pharmaceutical Industry.” Journal of Financial Economics, vol. 80, no. 2, 2006, pp. 351–383., doi:10.1016/j.jfineco.2005.04.004.
History.com Editors. “Dr. Jonas Salk Announces Polio Vaccine.” History.com, A&E Television Networks, 9 Feb. 2010, www.history.com/this-day-in-history/salk-announces-polio-vaccine.
Im, Ghiyoung, and Arun Rai. “Knowledge Sharing Ambidexterity in Long-Term Interorganizational Relationships.” Management Science, vol. 54, no. 7, 2008, pp. 1281–1296., doi:10.1287/mnsc.1080.0902.
Jones, Janet, and Michael Minor. “New, Strategic Outsourcing Models to Meet Changing Clinical Development Needs.” Perspectives in Clinical Research, vol. 1, no. 2, Medknow Publications Pvt Ltd, 2010, pp. 76–79, http://www.picronline.org/article.asp?issn=2229-3485;year=2010;volume=1;issue=2;spage=76;epage=79;aulast=Jones;type=0.
Julious, Steven A. “Sample Sizes for Clinical Trials.” 2009, doi:10.1201/9781584887409.
Kirk, Katie, and Lisa Hamelmann. “THE UNITED NATIONS SECRETARY-GENERAL’S HIGH-LEVEL PANEL ON ACCESS TO MEDICINES REPORT.” High-Level Panel on Access to Medicines, 14 Sept. 2016, www.unsgaccessmeds.org/final-report/.
Lahart, Justin. “Covid Is Crushing Small Businesses. That’s Bad News for American Innovation.” The Wall Street Journal, Dow Jones & Company, 9 Oct. 2020, www.wsj.com/articles/covid-is-crushing-small-businesses-thats-bad-news-for-american-innovation-11602235804.
Love, James. “Delinkage of R&D Costs From Product Prices.” Intellectual Property Watch, 15 Sept. 2016, www.ip-watch.org/2016/09/15/delinkage-of-rd-costs-from-product-prices/.
Love, James. “AN ECONOMIC PERSPECTIVE ON DELINKING THE COST OF R&D FROM THE PRICE OF MEDICINES.” UNITAID, 2016, www.unitaid.org/assets/Delinkage_Economic_Perspective_Feb2016.pdf.
Mannino, Gabrielle. “Maine CDC: 4,400 Moderna Vaccine Doses Compromised; US CDC, Operation Warp Speed Investigating.” Newscentermaine.com, 19 Jan. 2021, www.newscentermaine.com/article/news/health/coronavirus/vaccine/maine-cdc-4400-moderna-vaccine-doses-compromised-us-cdc-operation-warp-speed-investigating/97-92faf37e-2bf1-4792-8225-2f07eb93245e.
Maryam Kermanimojarad. “What Is the Impact of Patient Recruitment on Offshoring of Clinical Trials?” Life Sciences, Society and Policy, vol. 16, no. 1, BMC, 2020, pp. 1–12, doi:10.1186/s40504-020-00104-4.
McDonald, Alison M., et al. What Influences Recruitment to Randomised Controlled Trials? A Review of Trials Funded by Two UK Funding Agencies. 2011.
Moran, Mary, et al. “Neglected Disease Research and Development: How Much Are We Really Spending? (Policy Forum).” PLoS Medicine, vol. 6, no. 2, Public Library of Science, 2009, p. e1000030, doi:10.1371/journal.pmed.1000030.
Nicol, Dianne, and Olasupo Owoeye. “Using TRIPS Flexibilities to Facilitate Access to Medicines.” World Health Organization, World Health Organization, 27 June 2013, www.who.int/bulletin/volumes/91/7/12-115865/en/.
O’Donnell, Carl, and Michael Erman. “Pfizer to Outsource Some Drug Production, Focus on Coronavirus Vaccine.” Reuters, Thomson Reuters, 8 May 2020, www.reuters.com/article/us-health-coronavirus-pfizer-vaccine/pfizer-to-outsource-some-drug-production-focus-on-coronavirus-vaccine-idUSKBN22K2ZS.
O’Neill, Jim. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Dec. 2014, amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
Sandler, Rachel. Car Rental Company Hertz Files for Bankruptcy. 23 May 2020, www.forbes.com/sites/rachelsandler/2020/05/22/car-rental-company-hertz-files-for-bankruptcy/?sh=645954decfe9.
Shipman, Matt, and Dennis Orsi. “Vaccine Q&A: How Long Does It Take to Make Vaccines?” NC State News, 16 Dec. 2020, news.ncsu.edu/2020/12/vaccine-manufacturing-q-and-a/.
Spinner, Jenni. “Parexel, NeoGenomics Partner on Oncology Genomics Project.” Outsourcing, William Reed Business Media Ltd., 18 Feb. 2021, www.outsourcing-pharma.com/Article/2021/02/18/Parexel-NeoGenomics-partner-on-oncology-genomics-project.
Sully, Ben G. O., et al. “A Reinvestigation of Recruitment to Randomised, Controlled, Multicenter Trials: a Review of Trials Funded by Two UK Funding Agencies.” Trials, vol. 14, no. 1, BioMed Central, 2013, pp. 166–166, doi:10.1186/1745-6215-14-166.
Twin, Alexandra. “Why Companies Use Outsourcing.” Investopedia, Investopedia, 11 Mar. 2021, www.investopedia.com/terms/o/outsourcing.asp.
Wibulpolprasert, Suwit et al. “Government use licenses in Thailand: The power of evidence, civil movement and political leadership.” Globalization and health vol. 7 32. 12 Sep. 2011, doi:10.1186/1744-8603-7-32.
Witters, Dan. “Millions in U.S. Lost Someone Who Couldn’t Afford Treatment.” Gallup.com, Gallup, 23 Mar. 2021, news.gallup.com/poll/268094/millions-lost-someone-couldn-afford-treatment.aspx.
Wong, Hilary. “The case for compulsory licensing during COVID-19.” Journal of global health vol. 10,1 (2020): 010358. doi:10.7189/jogh.10.010358.